
The Greatest Law Firm You Can Trust
Oxbryta
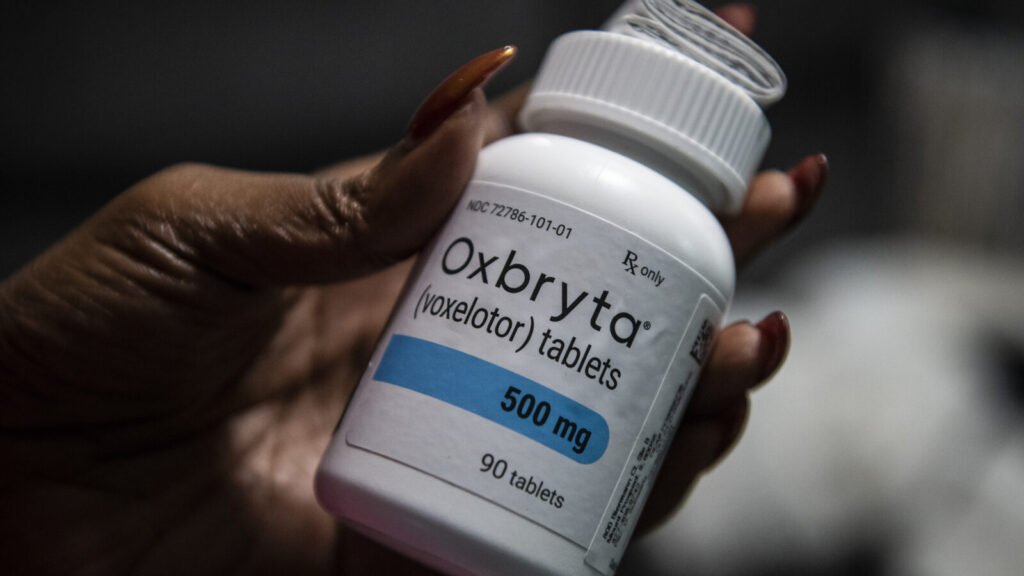
About Oxbryta
Following the September 2024 global withdrawal of Oxbryta (voxelotor) due to safety concerns, several lawsuits have been filed against its manufacturers, Pfizer and Global Blood Therapeutics (GBT). These legal actions allege that the companies failed to adequately warn patients and healthcare providers about the drug’s potential risks, including severe complications such as vaso-occlusive crises (VOCs), organ damage, and fatalities.
One notable case involves plaintiff Tirrell Allen, who began using Oxbryta in August 2024. After starting the medication, Allen reportedly experienced an increased frequency of VOCs, leading to severe pain and health complications. The lawsuit, filed in California, accuses the manufacturers of negligence and failure to provide adequate warnings about the drug’s risks
Patients who have experienced adverse effects from Oxbryta are advised to consult with legal professionals to explore their options for seeking compensation. Law firms specializing in pharmaceutical litigation are currently investigating potential claims related to Oxbryta’s alleged side effects and the circumstances surrounding its recall.
Please fill out the form below to receive detailed guidance on how to obtain compensation.
Fill The Details
Contact Info
- 258 8th Ave, New York, NY 10011, USA
- (+1) 3022084082
- contact@reclaimsspecialist.com
- 24 x 7
© 2021 | All Rights Reserved | The information provided on this site is not intended to be legal advice. For advice specific to your situation, please consult an attorney. We welcome your calls, letters, and emails, but contacting us does not establish an attorney-client relationship. Please refrain from sending confidential information until an attorney-client relationship has been formally established. We are admitted to practice in New Jersey, New York, Hawaii, California, Ohio, Minnesota, and Washington D.C., as well as before federal agencies and tribunals.
